What Chemicals Are In A Hot Pack . each hot pack contains: They are required to determine what chemical reaction. They typically last about thirty minutes but get reasonably warm. hot packs using calcium chloride are the bottle rockets of the hot pack world. the main chemical in single use hot packs is either calcium chloride or magnesium sulfate; There are a few ways that exothermic reactions can be used as hot packs. Many commercially available hot packs utilize common and safe chemicals to generate heat. in an endothermic reaction, a substance takes heat from its surroundings. The reaction, in this lab, mixes. Students design and build a reusable heat pack. hot packs take advantage of chemical reactions that produce heat as they progress. The sodium thiosulfate needs energy to.
from www.osfhealthcare.org
the main chemical in single use hot packs is either calcium chloride or magnesium sulfate; The reaction, in this lab, mixes. hot packs take advantage of chemical reactions that produce heat as they progress. hot packs using calcium chloride are the bottle rockets of the hot pack world. They typically last about thirty minutes but get reasonably warm. Many commercially available hot packs utilize common and safe chemicals to generate heat. each hot pack contains: They are required to determine what chemical reaction. There are a few ways that exothermic reactions can be used as hot packs. in an endothermic reaction, a substance takes heat from its surroundings.
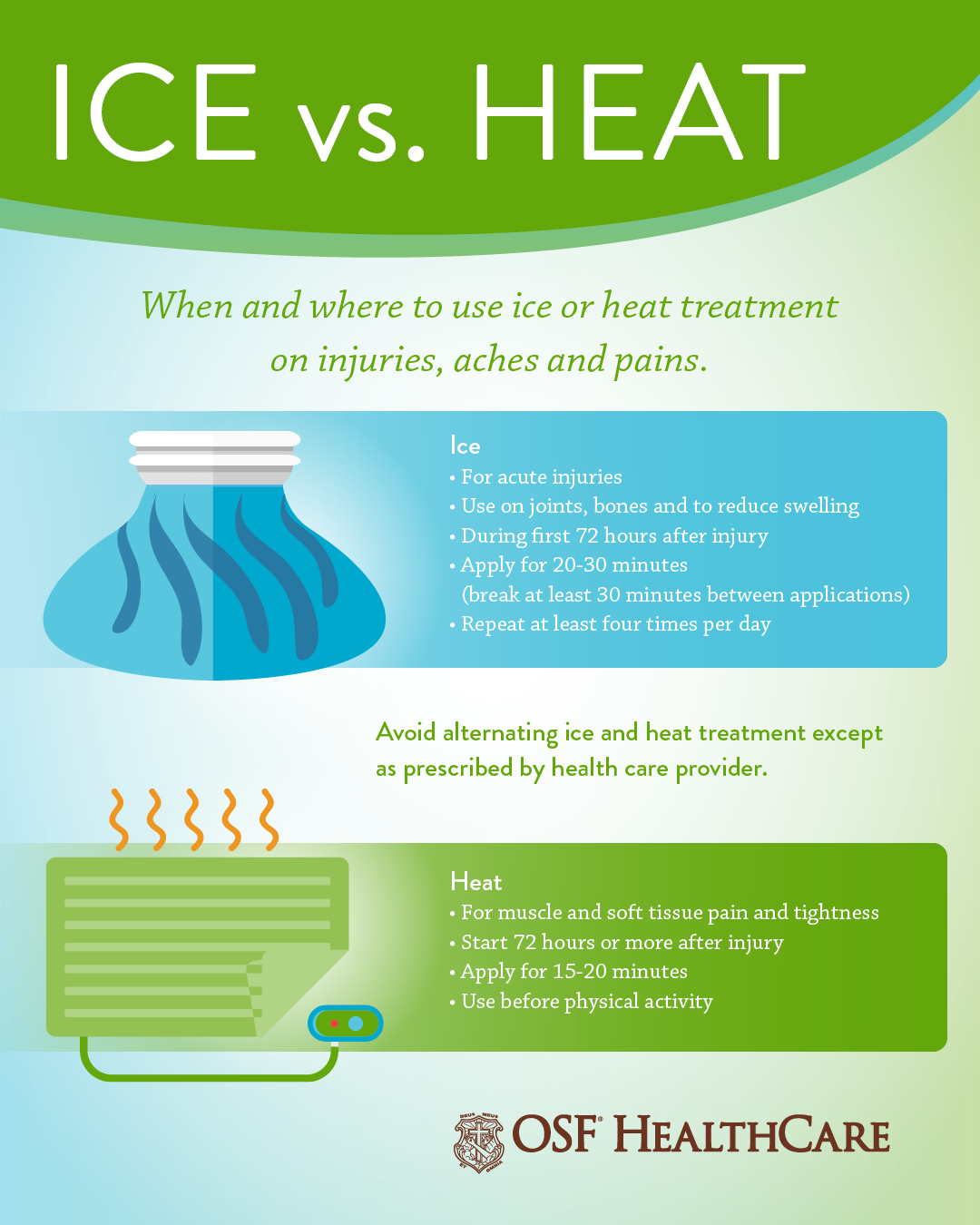
When to use ice or heat on an injury OSF HealthCare
What Chemicals Are In A Hot Pack The sodium thiosulfate needs energy to. They typically last about thirty minutes but get reasonably warm. hot packs using calcium chloride are the bottle rockets of the hot pack world. each hot pack contains: Students design and build a reusable heat pack. in an endothermic reaction, a substance takes heat from its surroundings. the main chemical in single use hot packs is either calcium chloride or magnesium sulfate; Many commercially available hot packs utilize common and safe chemicals to generate heat. hot packs take advantage of chemical reactions that produce heat as they progress. The sodium thiosulfate needs energy to. There are a few ways that exothermic reactions can be used as hot packs. The reaction, in this lab, mixes. They are required to determine what chemical reaction.
From nortechlabs.com
COOL Instant Cold Packs Nortech Labs Inc What Chemicals Are In A Hot Pack the main chemical in single use hot packs is either calcium chloride or magnesium sulfate; hot packs using calcium chloride are the bottle rockets of the hot pack world. The reaction, in this lab, mixes. They are required to determine what chemical reaction. They typically last about thirty minutes but get reasonably warm. Many commercially available hot packs. What Chemicals Are In A Hot Pack.
From www.rsa.global
Packaging of Chemicals What is it About? What Chemicals Are In A Hot Pack Students design and build a reusable heat pack. hot packs using calcium chloride are the bottle rockets of the hot pack world. the main chemical in single use hot packs is either calcium chloride or magnesium sulfate; They typically last about thirty minutes but get reasonably warm. Many commercially available hot packs utilize common and safe chemicals to. What Chemicals Are In A Hot Pack.
From studylib.net
Chem32a_Hot Pack_30oct13 What Chemicals Are In A Hot Pack They are required to determine what chemical reaction. Students design and build a reusable heat pack. each hot pack contains: hot packs using calcium chloride are the bottle rockets of the hot pack world. in an endothermic reaction, a substance takes heat from its surroundings. hot packs take advantage of chemical reactions that produce heat as. What Chemicals Are In A Hot Pack.
From shopee.com.my
🔥HOT🔥 Food Warmer Self Heating Pad Flameless Ration Heater / Heating What Chemicals Are In A Hot Pack hot packs take advantage of chemical reactions that produce heat as they progress. in an endothermic reaction, a substance takes heat from its surroundings. Many commercially available hot packs utilize common and safe chemicals to generate heat. They are required to determine what chemical reaction. The reaction, in this lab, mixes. each hot pack contains: Students design. What Chemicals Are In A Hot Pack.
From www.teachersource.com
Chemical Heat Pack Shop Our Chemical Heat Packs for Your Chemistry What Chemicals Are In A Hot Pack There are a few ways that exothermic reactions can be used as hot packs. Students design and build a reusable heat pack. hot packs using calcium chloride are the bottle rockets of the hot pack world. the main chemical in single use hot packs is either calcium chloride or magnesium sulfate; The reaction, in this lab, mixes. . What Chemicals Are In A Hot Pack.
From cpr-1staid.com
Chemical Hot Pack CPR 1st Aid What Chemicals Are In A Hot Pack They typically last about thirty minutes but get reasonably warm. in an endothermic reaction, a substance takes heat from its surroundings. hot packs take advantage of chemical reactions that produce heat as they progress. The sodium thiosulfate needs energy to. The reaction, in this lab, mixes. They are required to determine what chemical reaction. There are a few. What Chemicals Are In A Hot Pack.
From www.teachersource.com
Chemical Heat Pack Shop Our Chemical Heat Packs for Your Chemistry What Chemicals Are In A Hot Pack The sodium thiosulfate needs energy to. Students design and build a reusable heat pack. They typically last about thirty minutes but get reasonably warm. in an endothermic reaction, a substance takes heat from its surroundings. The reaction, in this lab, mixes. hot packs using calcium chloride are the bottle rockets of the hot pack world. Many commercially available. What Chemicals Are In A Hot Pack.
From www.osfhealthcare.org
When to use ice or heat on an injury OSF HealthCare What Chemicals Are In A Hot Pack They typically last about thirty minutes but get reasonably warm. the main chemical in single use hot packs is either calcium chloride or magnesium sulfate; The reaction, in this lab, mixes. They are required to determine what chemical reaction. hot packs take advantage of chemical reactions that produce heat as they progress. in an endothermic reaction, a. What Chemicals Are In A Hot Pack.
From sciencing.com
Chemicals Used in Heat Packs Sciencing What Chemicals Are In A Hot Pack The reaction, in this lab, mixes. Students design and build a reusable heat pack. the main chemical in single use hot packs is either calcium chloride or magnesium sulfate; in an endothermic reaction, a substance takes heat from its surroundings. The sodium thiosulfate needs energy to. There are a few ways that exothermic reactions can be used as. What Chemicals Are In A Hot Pack.
From www.ellenjai.com
最新人気 スーパーウォーマー ボディ&ハンド 特別価格HotHands 長持ち 40個の個別ウォーマ好評販売中 最大18時間の熱 What Chemicals Are In A Hot Pack in an endothermic reaction, a substance takes heat from its surroundings. They are required to determine what chemical reaction. The reaction, in this lab, mixes. Students design and build a reusable heat pack. Many commercially available hot packs utilize common and safe chemicals to generate heat. hot packs using calcium chloride are the bottle rockets of the hot. What Chemicals Are In A Hot Pack.
From thewonderofscience.com
Reusable Heat Packs — The Wonder of Science What Chemicals Are In A Hot Pack in an endothermic reaction, a substance takes heat from its surroundings. hot packs take advantage of chemical reactions that produce heat as they progress. Students design and build a reusable heat pack. hot packs using calcium chloride are the bottle rockets of the hot pack world. They are required to determine what chemical reaction. each hot. What Chemicals Are In A Hot Pack.
From exozasipi.blob.core.windows.net
How Hot Should A Heat Pack Be at Jean Stewart blog What Chemicals Are In A Hot Pack The reaction, in this lab, mixes. in an endothermic reaction, a substance takes heat from its surroundings. They are required to determine what chemical reaction. Many commercially available hot packs utilize common and safe chemicals to generate heat. each hot pack contains: The sodium thiosulfate needs energy to. Students design and build a reusable heat pack. There are. What Chemicals Are In A Hot Pack.
From www.chinookmed.com
Disposable Heat Pack Chinook Medical Gear What Chemicals Are In A Hot Pack They typically last about thirty minutes but get reasonably warm. each hot pack contains: There are a few ways that exothermic reactions can be used as hot packs. The reaction, in this lab, mixes. hot packs take advantage of chemical reactions that produce heat as they progress. Students design and build a reusable heat pack. in an. What Chemicals Are In A Hot Pack.
From arenahanna.wordpress.com
HEAT WORLD OF PHYSICS steps by steps to understand heat What Chemicals Are In A Hot Pack Many commercially available hot packs utilize common and safe chemicals to generate heat. in an endothermic reaction, a substance takes heat from its surroundings. They typically last about thirty minutes but get reasonably warm. They are required to determine what chemical reaction. The sodium thiosulfate needs energy to. each hot pack contains: Students design and build a reusable. What Chemicals Are In A Hot Pack.
From www.inflatablehottubguide.com
The UK's Best Hot Tub Chemicals to Use on a Garden Spa 2022 What Chemicals Are In A Hot Pack each hot pack contains: in an endothermic reaction, a substance takes heat from its surroundings. The reaction, in this lab, mixes. There are a few ways that exothermic reactions can be used as hot packs. hot packs take advantage of chemical reactions that produce heat as they progress. Students design and build a reusable heat pack. Many. What Chemicals Are In A Hot Pack.
From gioericiw.blob.core.windows.net
What Chemicals Are In A Heat Pack at Valorie Smith blog What Chemicals Are In A Hot Pack each hot pack contains: Students design and build a reusable heat pack. The sodium thiosulfate needs energy to. in an endothermic reaction, a substance takes heat from its surroundings. They typically last about thirty minutes but get reasonably warm. They are required to determine what chemical reaction. Many commercially available hot packs utilize common and safe chemicals to. What Chemicals Are In A Hot Pack.
From gioericiw.blob.core.windows.net
What Chemicals Are In A Heat Pack at Valorie Smith blog What Chemicals Are In A Hot Pack They are required to determine what chemical reaction. They typically last about thirty minutes but get reasonably warm. each hot pack contains: Many commercially available hot packs utilize common and safe chemicals to generate heat. hot packs using calcium chloride are the bottle rockets of the hot pack world. in an endothermic reaction, a substance takes heat. What Chemicals Are In A Hot Pack.
From www.uslrgp.com
Hot Pack Instant Chemical Activation General Purpose 5 X 9 Inch US What Chemicals Are In A Hot Pack the main chemical in single use hot packs is either calcium chloride or magnesium sulfate; There are a few ways that exothermic reactions can be used as hot packs. Many commercially available hot packs utilize common and safe chemicals to generate heat. in an endothermic reaction, a substance takes heat from its surroundings. hot packs take advantage. What Chemicals Are In A Hot Pack.